Drug Treatment and Radiotherapy for Melanoma
Traditional Drug Treatments for Metastatic Melanoma (Stage IV Disease)
Until March 2011, when Ipilimumab was approved by the FDA (The US Food and Drug Administration) for treating metastatic melanoma, the only treatments available for stage IV metastatic melanoma were chemotherapy and/or radiotherapy.
Single-agent chemotherapy drugs that have been shown to work for metastatic melanoma are Dacarbazine and Temozolomide (the tablet form of Dacarbazine). Combinations of drugs such as Paclitaxel and Carboplatin have also been used. The problem with chemotherapy is that the response rate is only about 15-20% for single agents and about 40% for combination treatment and only a very small percentage of patients who respond get any lasting benefit.
Chemotherapy drugs are no longer first line therapy for melanoma metastases and have been replaced by other classes of drugs that either stimulate the immune system or directly block mutated enzymes in melanoma cells. Chemotherapy is now reserved for patients where all the newer treatment options have been exhausted.
Preface to the New Drug Treatments for Melanoma
All the new treatments for metastatic melanoma are very expensive and most are not fully covered (or not at all) by medical aids. Only three of the nine new melanoma drugs have been fully registered for use in South Africa. They are Vemurafenib, Pembrolizumab and Ipilimumab. A further four drugs are available through a Section 21 application to the Medicines Control Council (one can apply to bring drugs into the country that are registered in other countries but not South Africa). These drugs are Dabrafenib, Trametinib, Nivolumab and Cobimetinib. The average cost of each drug is about R1 million to R1.5 million per year. The optimal lengths of treatment for each of the drugs has not been established so no one knows how long they need to be taken. It may be necessary to continue taking these drugs for as long as they are working, which may be years, and this clearly has some major cost implications. Some of these new drugs are most effective when used in combination. So instead of R1 million to R1.5 million per year the cost doubles to R2 million to R3 million per year.
Some of these drugs have a lot of side effects which force people to stop taking them and the number of side effects often increases when one combines drugs. Otherwise, the new drugs for treating metastatic melanoma have been able to produce some spectacular results and have brought a lot of hope to many people. There is evidence that some patients may actually be cured of metastatic melanoma by these new drugs.
Research is ongoing and is actually accelerating in the field of biological drugs, so one would predict that many new, more effective and possibly cheaper drugs may be available in the coming years.
Summary of the New Drug Treatments for Melanoma
There are two main groups of drugs (all discussed in detail below).
- Chemical drugs that block mutated enzymes in melanoma cells. The mutated enzymes send signals for the melanoma cells to divide and spread. When the enzymes are switched off, the melanoma cells die.
- Vemurafenib (approved by the FDA in August 2011)
- Dabrafenib (approved by the FDA in May 2013)
- Combination therapy with Dabrafenib and Trametinib (approved by the FDA in January 2014)
- Combination therapy with Vemurafenib and Cobimetinib (approved by the FDA in November 2015)
- Combination therapy with Encorafenib and Binimetinib (approved by the FDA in June 2018)
- Antibodies that stimulate the body's own immune system to kill melanoma cells.
- CTLA-4 inhibitors — Ipilimumab (approved by the FDA in March 2011)
- PD-1 inhibitors — Nivolumab (approved by the FDA in December 2014)
and Pembrolizumab (approved by the FDA in September 2014) - Combination therapy with both Nivolumab and Ipilimumab (approved by the FDA in October 2015)
Chemical Drugs
The following discussion is necessarily quite detailed but gives enough information to be able to understand how the chemical blocking drugs listed above work. They all function in similar ways by blocking mutated enzymes in melanoma cells that are driving cell multiplication.
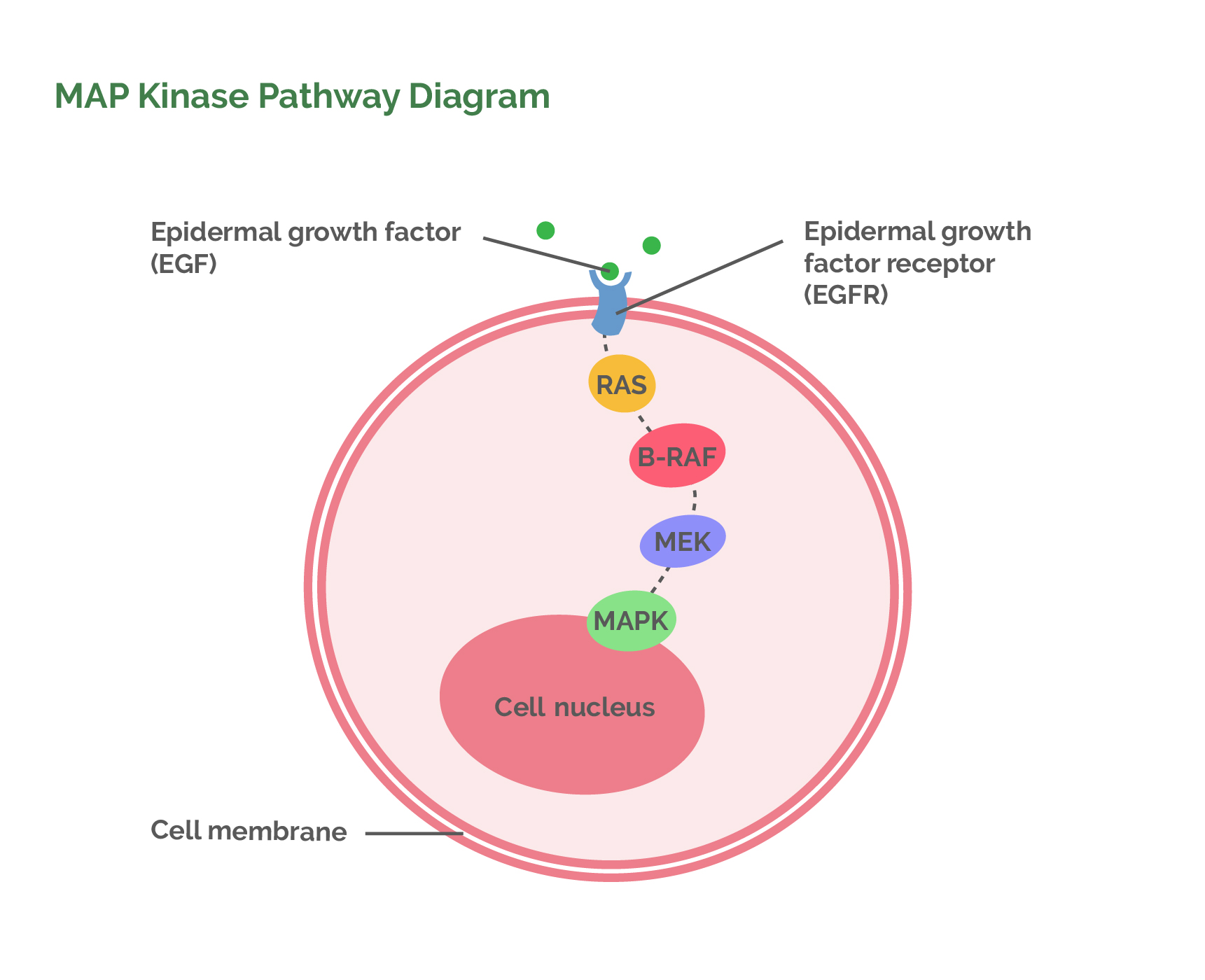
Normal cell division is initiated by a well-described mechanism where a specific stimulation molecule (EGF) attaches to the surface of a cell. This activates an enzyme (RAS) that sends a signal to another enzyme (RAF) within the cell. This enzyme then switches on another enzyme (MEK) which switches on another until finally, at the end of this chain reaction, a protein (MAPK)is produced that then attaches to the DNA to initiate replication. The chain reaction of enzymes which results in the division of a cell is known as the MAP Kinase pathway. MAP stands for Mitogen Activated Protein. A kinase is an enzyme that transfers a phosphate group from ATP to a specific molecule to activate that molecule. What follows is an abbreviated description of the MAP Kinase pathway relevant to melanoma and the new chemical drugs.
An important concept to understand is that if any enzyme in the MAP Kinase pathway mutates and becomes overactive it will over-stimulate all the other enzymes further along the pathway. This results in rapid and continuous cell division and the formation of a cancerous growth. The knowledge that this is one of the main mechanisms which cause cancers to grow has allowed researchers to look for ways of switching off the mutated cancer enzymes.
MAP Kinase Pathway
(Please refer to the flow diagram.)
- Cell replication (creating new cells) is initiated when EGF (Epidermal Growth Factor, a hormone in the blood) binds to EGFR (Epidermal Growth Factor Receptor, an enzyme in the membrane of the cell that is being stimulated to divide). EGF switches on EGFR which can then activate the next enzyme in the chain which is RAS.
- The active EGFR turns on the RAS enzyme.
- The active RAS enzyme then switches on the RAF enzyme. Two types of RAF have subsequently been found, labelled alphabetically as A-RAF and B-RAF. B-RAF is specific to melanoma.
- Active B-RAF then stimulates the MEK enzyme which, in turn, stimulates MAPK (Mitogen Activated Protein Kinase).
- The MAPK enzyme gives its name to the pathway because it is the crucial enzyme that enters the cell nucleus to activate proteins which bind to DNA to start cell replication.
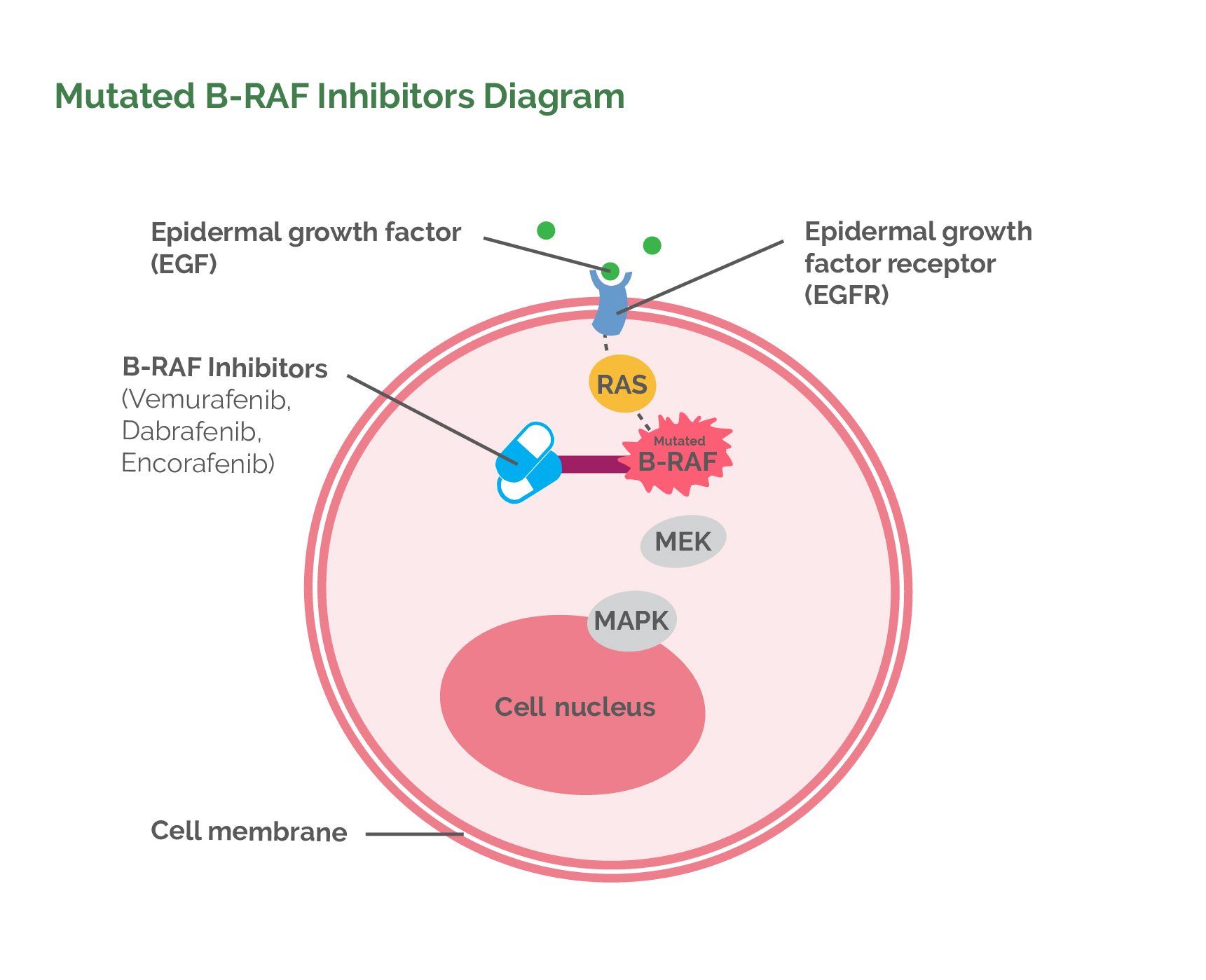
Mutated B-RAF Inhibitors
-
Vemurafenib
In 2002, it was discovered that the most common mutated enzyme in the MAPK pathway in melanomas is B-RAF, occurring up to 45% of the time. Research was then initiated to develop a drug to block the activity of the mutated B-RAF enzyme. The culmination of this research was the production of a chemical drug called Vemurafenib, which was approved by the FDA in August 2011. It is marketed as Zelboraf. Vemurafenib binds to the mutated B-RAF enzyme and stops it from working. The important thing is that this doesn’t just stop cell division it actually results in the death of that melanoma cell. Another very important concept is that Vemurafenib can only bind to and inactivate mutated B-RAF enzymes. It therefore does not interrupt normal cell division in healthy cells. However, it does not work in melanomas without the B-RAF mutation. To decide whether a patient is eligible to receive Vemurafenib, some tissue from their melanoma (the original melanoma or from a metastasis) is tested to see if the B-RAF mutation is present or not.Vemurafenib is taken as a tablet twice a day. About 50% of patients show either complete or partial disappearance of their metastases. The other 50% have no response. Unfortunately, a large percentage of patients who do initially respond to Vemurafenib relapse because the melanoma cells become resistant to the drug. Methods of primary and secondary resistance are being researched to allow ways of producing a greater response to Vemurafenib. About 20% of people, however, do continue to have a long term response to Vemurafenib and it is starting to appear as though some people may actually be cured of their metastases. Research is ongoing. Vemurafenib does have a long list of side effects, although, it is generally well tolerated. The worst side effects relate to skin rashes and activation of latent skin cancers.
- Dabrafenib
Dabrafenib is another B-RAF enzyme inhibitor drug which is available on the American and European markets. It was approved by the FDA in May 2013 for treatment of metastatic melanoma. Dabrafenib has a similar response rate and side-effect profile to Vemurafenib. - Encorafenib
Encorafenib is a B-RAF inhibitor that has a higher affinity for B-RAF and a longer binding time with B-RAF than other B-RAF inhibitors. It has been evaluated in combination with Binimetinib, a MEK inhibitor (see below). Results showed that there was a longer period of time before melanoma recurred (14,9 months) with this combination when compared to Vemurafenib alone (7,3 months). Based on this, the combination was approved by the FDA in June 2018.
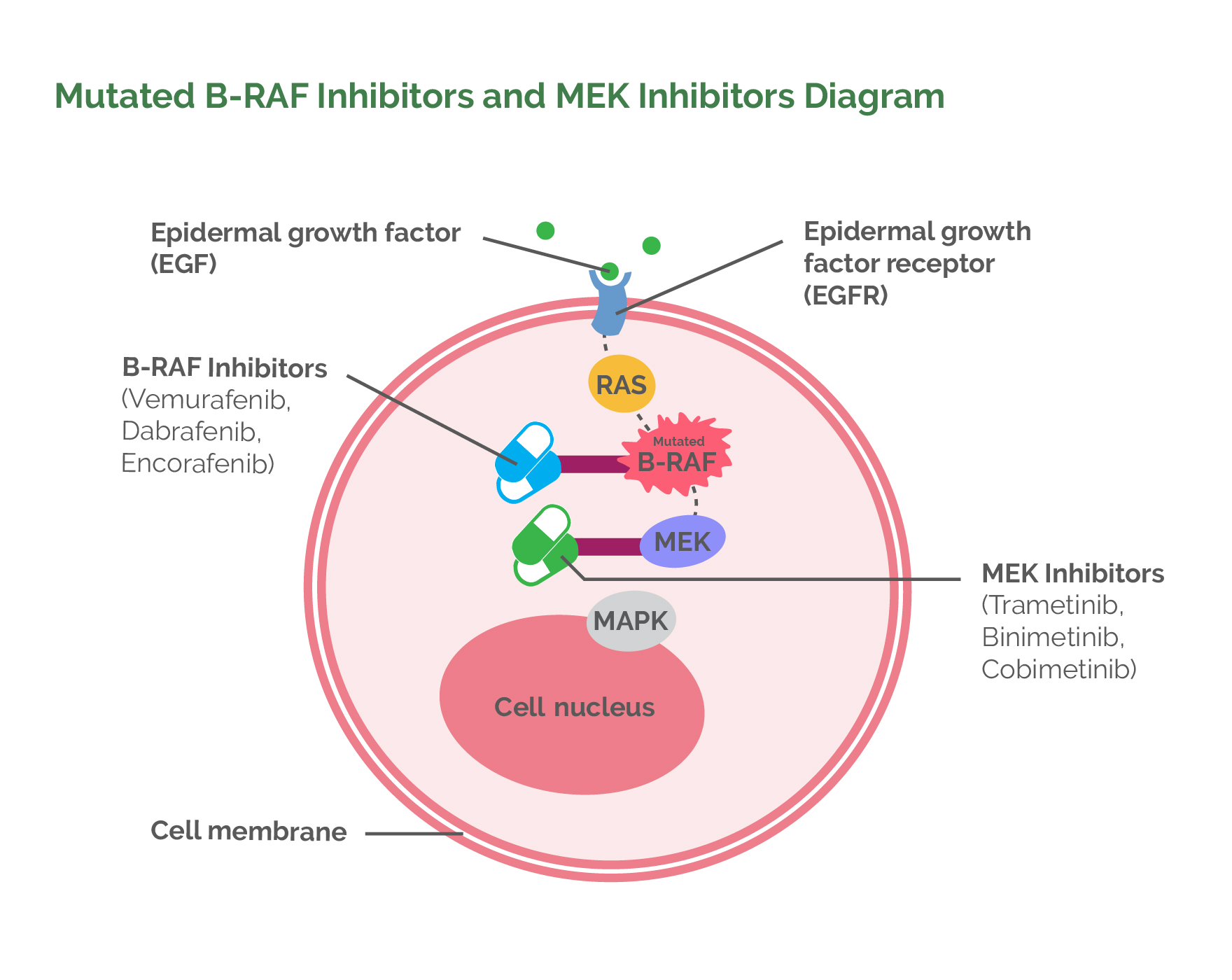
Drugs that Inhibit the MEK Enzyme
In May 2013, the FDA released Trametinib for the treatment of metastatic melanoma. This is a chemical that switches off normally-functioning MEK enzymes (the enzyme that B-RAF proteins directly stimulate). In patients who have mutated B-RAF, switching off the MEK enzyme prevents further stimulation of cell division.
In January 2014, the FDA approved combination therapy using both a B-RAF inhibitor (Dabrafenib) and a MEK inhibitor (Trametinib). This combination increased the response rate (i.e. metastases disappearing or significantly reducing in size) to 64% when compared to Vemurafenib alone (Vemurafenib alone has a response rate of 50%) and resulted in a significantly higher overall survival rate at one year (72% vs 65%) when compared to using Vemurafenib alone. At three years, 43% of the responders to the Dabrafenib/Trametinib combination were still alive. Importantly, the combination of the two drugs did not result in a greater number of side effects when compared to using Vemurafenib alone. So, at present the gold standard for treating people who have metastatic melanoma that cannot be removed surgically and whose melanoma has the B-RAF mutation is to use a combination of Dabrafenib (B-RAF inhibitor) and Trametinib (MEK inhibitor).
As mentioned above, Encorafenib has been combined with Binimetinib. Vemurafenib has also been combined with the MEK inhibitor Cobimetinib and this combination was approved by the FDA in November 2015.
Other Enzyme Mutations in the MAPK Pathway that can be Blocked
There has been a lot of research done to find drugs which block the other two main enzymes in the MAPK pathway, namely RAS and MAPK. So far there has been no success and research is ongoing.
Treatments for Patients Who Don't Respond to or Are Not Candidates for B-RAF and MEK Inhibitors
55% of patients do not have B-RAF mutations and only 64% of patients with B-RAF mutations initially respond to treatments. This means that overall, only 29% of all patients will respond to treatment with B-RAF and MEK inhibitors. In addition, a significant percentage of these patients will relapse within a year of starting treatment. Fortunately, there is another category of drugs available for treating these patients.
Antibodies that Stimulate the Body's Immune System to Fight Cancer
For years it has been known that our immune system can fight cancer cells. Usually, the immune system’s initial response to the formation of a cancer is a brisk attack, but most commonly this attack quickly fades in intensity before all the cancer cells have been destroyed, and this allows the cancer to continue growing. After many years of research into this phenomenon, it was found that the body itself actively switches off the immune system response to cancer. Discovering the mechanisms behind this has made it possible to develop drugs that stimulate the immune system to kill cancer cells.
The Basic Workings of the Immune System
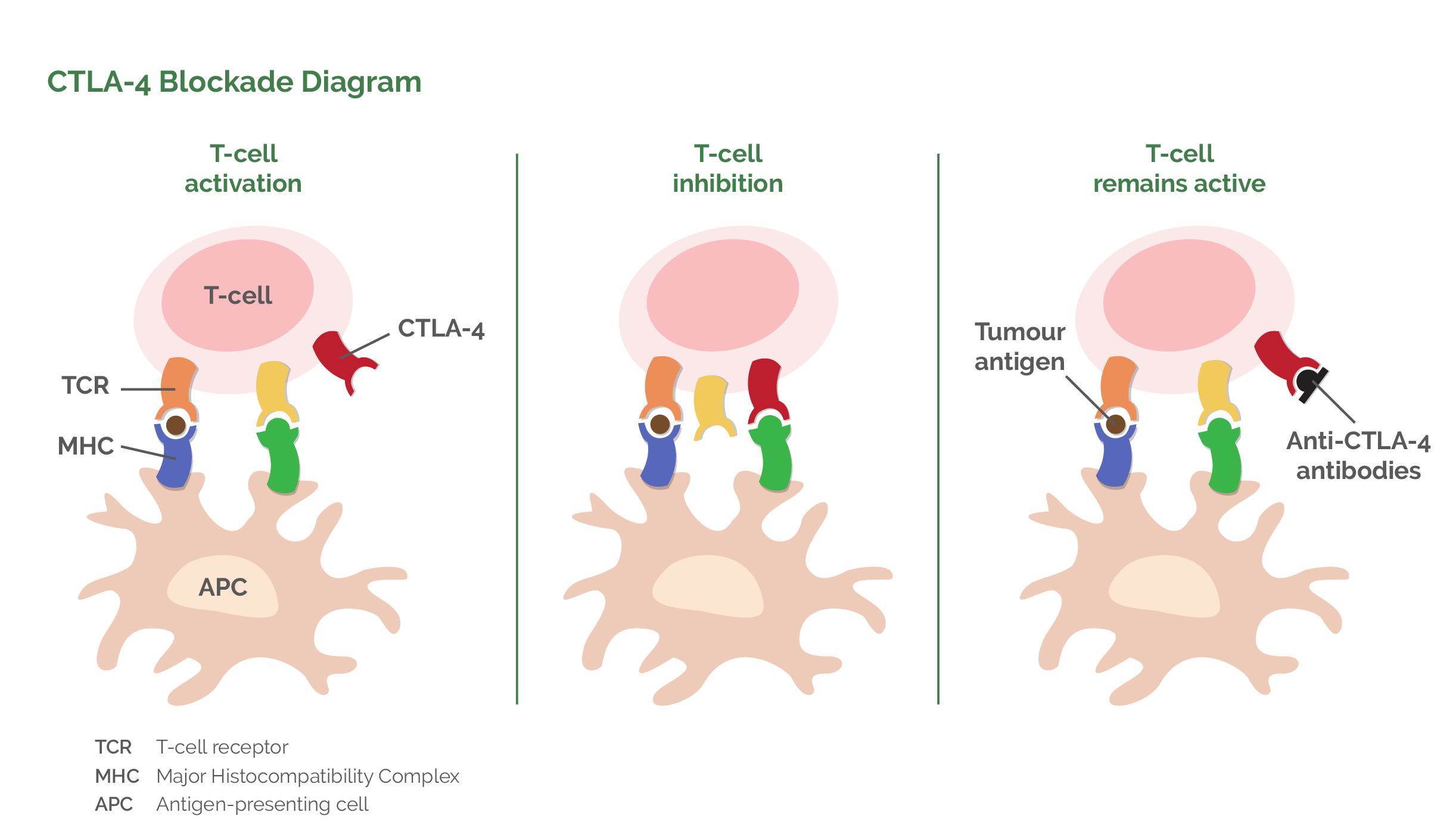
The main cells of the immune system that are responsible for killing cancer cells are called T-lymphocytes. They get their name from the fact that they are mostly found in lymph tissue. They are called T-lymphocytes because they mature in the Thymus gland in the neck. T-lymphocytes cannot immediately recognise cancer cells and therefore have to first be “taught” to do so by other cells. The cells that do the teaching are called accessory or antigen-presenting cells. These are very large (but not very numerous) cells that have an innate ability to recognise tumour cells as being foreign. When an accessory cell encounters a cancer cell it is able to ingest (phagocytose) this cell and then destroy it by enzymatically breaking it up into lots of little particles (called antigens). The accessory cell then takes the tumour particles (antigens) and pushes them through onto the surface of its cell membrane where they become attached to proteins called MHC proteins (Major Histocompatibility Complex proteins). The accessory cell then shows or “presents” the tumour particles (antigens) combined with the MHC proteins to the T-Lymphocytes (hence the accessory cell’s other name of antigen-presenting cell). In this way, it shows the T-lymphocytes parts of the destroyed cancer cell and thus “teaches” the T-lymphocytes to recognise the cancer cells that need to be killed. The T-lymphocyte actually binds to the surface of the accessory cell for this process to happen. Once released from the accessory cell, the T-lymphocyte has become activated to go and hunt for cancer cells, which it kills by ingestion (phagocytosis).
An interesting thing happens when the T-lymphocyte is attached to the accessory cell. As well as activating the T-lymphocyte cell, the accessory cell also initiates a process to begin inactivation of the T-lymphocyte. It does this by switching on a protein on the T-lymphocyte surface called CTLA-4 (Cytotoxic T-lymphocyte-Antigen 4). Initially this CTLA-4 (off switch) is not very active but over time it becomes moreso until it is so active that the T-lymphocyte switches off. The body does this as a control mechanism so that there is a way of regulating the active T-lymphocyte cells. Unfortunately, the switch-off mechanism is often premature and occurs before all the cancer cells have been killed. Once this switch-off mechanism had been identified, intense research resulted in the production of a drug which allowed the switching-off mechanism to be blocked. An antibody was produced which binds to the CTLA-4 (off switch) molecule prior to T-lymphocyte binding to the accessory cell. This thus prevents the CTLA-4 (off switch) from being activated by the accessory cell. T-lymphocytes thus become active for much greater periods of time and can kill a far larger number of cancer cells. The name given to the antibody blocker is Ipilimumab.
Further research has found that active T-lymphocytes have yet another surface molecule that allows them to be switched off. The molecule is called PD1 (Programmed Death receptor 1). Healthy human cells need to be able to protect themselves from active T-lymphocytes so they all have a protein on their cell surfaces called PD-L (Programmed cell Death Ligand). When an active T-lymphocyte comes along and tries to attack a healthy cell, the healthy cell binds its PD-L molecule with the active T-lymphocyte PD1 molecule and the T-lymphocyte gets switched off. The unfortunate thing is that many cancer cells also carry the PD-L molecule. They can therefore also inactivate the T-lymphocytes and protect themselves from destruction. Science has again stepped in to produce an antibody which, when given intravenously, attaches to the PD1 receptor on the surface of the T-lymphocyte. The function of the antibody is to get in the way and make it impossible for the PD-L molecule on cancer cells to attach to the PD1 receptor on the T-lymphocyte. The active T-lymphocyte therefore cannot be switched off. There are two antibodies that have been produced to block PD1 receptors. These are known as Nivolumab and Pembrolizumab.
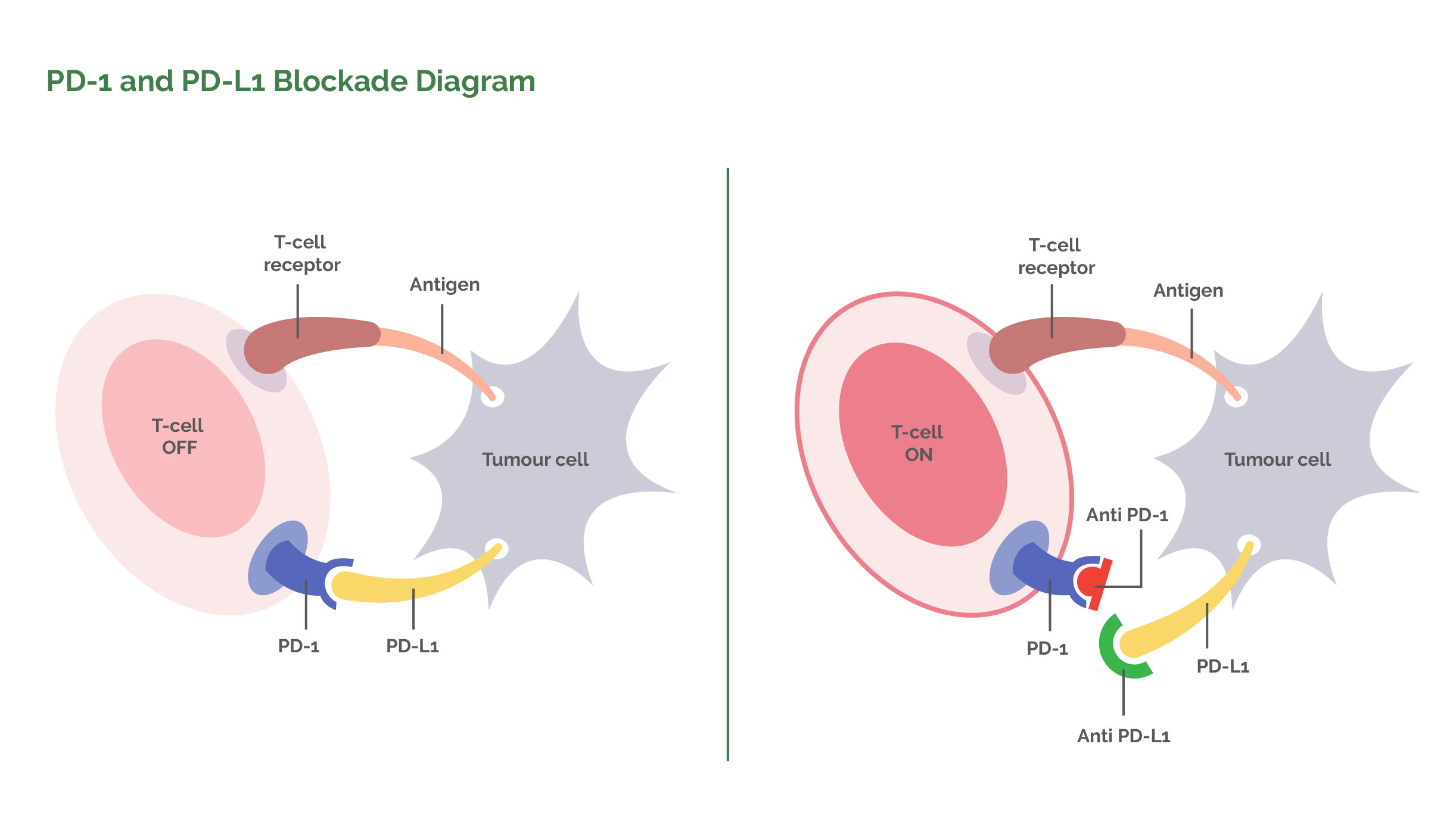
Ipilimumab (Ipi)
- This is a CTLA-4 blocker (blocks the off switch).
- Approved by the FDA in March 2011 for the treatment of metastatic melanoma.
- Sold under the trade name Yeroy.
- One of the big problems with Ipilimumab is that only about 10% of patients have a response to the drug.
- Another problem with Ipilimumab is that about 27% of patients experience severe side effects.
- The good news, however, is that some patients appear to have been cured by Ipilimumab with a survival rate of 9% at ten years. So far, no other treatment for metastatic melanoma can boast such good results.
- Ipilimumab is given as an intravenous infusion initially once every three weeks for four times and the process can be repeated if there is progression of the disease more than three months after an initial response.
Pembrolizumab (Pembro)
- This was the first of the PD1 inhibitors to be approved by the FDA for treating metastatic melanoma. Approval for use was given by the FDA in September 2014.
- It is sold under the brand name Keytruda.
- The average number of patients who respond to the drug is about 34% (compared with 10% for Ipilimumab).
- Of the 34% who respond initially, 44% will still be alive at the end of four years (54% at three years).
- There are far fewer and less severe side effects when compared to Ipilimumab.
- Severe side effects occur in 16% of patients.
- Pembrolizumab is given as an intravenous infusion.
Nivolumab (Nivo)
- This PD1 blocker was approved for use by the FDA in December 2014, only a few months after Pembro had been given the green light.
- Trials have shown that the average number of patients to show a response to the drug is about 44% and that 46% of responders will still be alive four years later (52% at three years), which is very similar to Pembrolizumab.
- Nivolumab also has fewer side effects than Ipilimumab, but about the same as Pembrolizumab.
- Overall, Nivolumab is marginally more effective than Pembrolizumab.
- Nivo is given as an intravenous infusion.
Combination Nivolumab and Ipilimumab
- It makes sense to combine the use of a PD1 blocker and a CTLA-4 blocker, i.e. use Ipilimumab and Nivolumab or Pembrolizumab at the same time. Both T-lymphocyte off-switches are then blocked simultaneously.
- One study has assesed this using Nivolumab and Ipilimumab. The response rate to this combination of drugs was 58% (which is higher than Nivo alone (44%), Pembro alone (34%), and Ipilimumab alone (19%)). Four year results are now out and they show that 53% of responders will still be alive (58% at three years). This is clearly better than the four year responses for Nivo (46%) and Pembro (44%). The combination of Nivo and Ipi has become the gold standard of treatment for immune stimulation.
- The big problem with combination therapy, however, is that side effects are severe in about 55% of patients and a significant number of people have to reduce doses or stop taking Ipilimumab.
- In September 2015, the FDA gave approval for the use of combination of Nivolumab and Ipilimumab for metastatic melanoma.
Adjuvant Therapy
Adjuvant therapy is defined as giving a treatment for cancer where there is no cancer detectable on screening. Adjuvant therapy is given to patients who have a high risk of developing future metastases, either because their original cancer was at an advanced stage or because they have already had metastases which have been surgically removed. Adjuvant therapy is used in melanoma patients who have had spread of melanoma either to their regional lymph nodes or an organ (lung, brain, liver) surgically resected and where there is no sign of spread of melanoma anywhere else (usually based on a clear PET scan).
The aim of adjuvant therapy is to kill any microscopic cancer cells thus preventing them from growing and thereby curing the patient. Until recently, there was no adjuvant treatment available for melanoma. Nothing had been shown to work. In May 2015, however, a paper was published showing that adjuvant Ipilimumab (at a higher dose than that used in the metastatic setting) given to melanoma patients with surgically resected lymph node metastases significantly reduced the rate of subsequent metastases. At three years, 46,5% of those who were given Ipilimumab were still clear of melanoma, while this was only true of 34,8% of patients who had received the placebo. In October 2015, the FDA approved Ipilimumab as an adjuvant drug for treating melanoma.
Nivolumab has now been compared with Ipilimumab for adjuvant therapy in patients with spread of melanoma to lymph nodes that have been surgically resected and patients with a systemic metastasis that has been surgically removed. The news is good. After two years, 63% of the patients on Nivolumab are still alive compared with 50% of patients taking Ipilimumab. Nivoulumab was approved by the FDA in December 2017. Pembrolizumab performs as well as Nivolumab in the adjuvant setting so can be used instead if Nivo is not available (South Africa). Pembrolizumab was approved by the FDA in June 2018 for adjuvant melanoma treatment.
Adjuvant therapy, using B-RAF and MEK inhibitors for patients who have the B-RAF mutation, has been studied as well. The combination of Dabrafenib and Trametinib was approved by the FDA in April 2018. Results at four years show a relapse-free survival (people who are alive who have no sign of any melanoma recurrence) of 54% for those taking the combination versus 38% relapse-free survival for patients taking a placebo.
Radiotherapy for Melanoma
Why do I Need Radiation Treatment After My Melanoma Surgery?
Radiation can be used in these instances:
Radiation can be used after surgery to reduce the risk of melanoma reoccurring.
Melanomas that are in a surgically difficult place that cannot be removed with adequate safety margins require radiation treatment after surgery.
Patients sometimes have to have lymph nodes removed from certain areas, such as the groin or under the arm. When melanoma is found in many lymph nodes or found growing outside of the lymph node (extracapsular spread), then patients require radiation to the area to reduce the chance of melanoma reoccurring. This radiation does not improve survival but rather helps control regrowth of melanoma in the area.
Radiation also plays an important role in relief of symptoms in patients who have advanced or metastatic disease (where the cancer has spread to other sites within the body). It can be used for pain relief or to reduce pressure on parts within one’s body or to reduce bleeding.
Radiation is generally given on a daily basis on weekdays and takes about 15 to 20 minutes to administer and the treatment is aimed and targeted at the area of interest.
Information as of January 2019